About Us
Deploying a first-in-class technology to enable best-in-class ophthalmic medicines.
Valitor is initially focused on improving patient outcomes in ophthalmology and vision-threatening diseases by conquering long-standing limitations of traditional biologic medicines. By leveraging its multivalent biopolymer technology, the company can take bioactive molecules and engineer best-in-class medicines with extended therapeutic durability. Valitor is using proven targets for ocular diseases to advance best-in-class medicines that maximize the clinical impact for patients.
Durable treatment for vision-threatening diseases
Long-acting VLTR-559 being developed for wet AMD
Interchangeable platform for efficient pipeline
Our Team
We have brought together seasoned biotechnology executives, innovative bioengineers and scientists, and leading drug developers to develop better medicines for patients.
Management
Gregory D. Kunst
Chief Executive Officer

Gregory D. Kunst is the chief executive officer at Valitor. Prior to Valitor, Greg served as chief executive officer and board member at Aurion Biotech, a private clinical-stage cell therapy company. Before Aurion, Greg spent six years at Glaukos Corporation, where he led the worldwide marketing, market access, reimbursement, health economics and outcomes research, government affairs, and business development teams. During his tenure at Glaukos, Greg launched four new products and led the development for multiple ophthalmology device, pharmaceutical and biologic candidates targeting corneal, glaucoma, and retinal diseases. Prior to Glaukos, Greg was global franchise director over the glaucoma surgery and retina pharmaceutical businesses at Alcon, a Novartis company. Prior to Alcon, Greg was the global head of market access at Kinetic Concepts, Inc. (Acelity Inc.) Greg is a national advisory board member of the Brigham Young University School of Life Sciences. Greg has previously been a member of the steering committee of the Gavin Herbert Eye Institute at the University of California, Irvine. Greg is an independent board member of a number of life sciences companies including Biomimetix, Pr3vent, Feliqs, and SIME Diagnostics. Greg holds an MBA from Vanderbilt University and a BS in Economics from Brigham Young University.
Wesley Jackson, PhD
President, Chief Scientific Officer & Member of the Board

Wesley Jackson, Ph.D., has been actively involved in biotechnology entrepreneurship for over 20 years as a scientist, researcher, and innovator. Dr. Jackson was part of Valitor's founding team and helped to in-license the novel protein-biopolymer conjugation technology, which now forms the basis of Valitor’s MVP platform, from U.C. Berkeley. Since launching Valitor, he has been the principal investigator on nine NIH projects to optimize the manufacturing, characterization, and formulation of multivalent protein conjugate drugs and to evaluate their performance in relevant disease models. Prior to Valitor, Dr. Jackson was a consultant for biotech startups providing expertise in establishing and implementing preclinical and clinical development plans. Prior to his industry tenure, he was a scientist and researcher affiliated with several universities, including U.C. Berkeley, and governmental agencies, such as National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Jackson has a Ph.D. in Bioengineering from the U.C. San Francisco and U.C. Berkeley, an M.S. in Bioengineering from U.C. San Francisco, and a B.S. in Bioengineering from U.C. Berkeley.
William S. (Sandy) White, PharmD, MBA
Executive Development Advisor

Sandy White, R.Ph., M.B.A., has over 35 years of broad pharmaceutical industry experience including general management, business development, research and development, and sales and marketing. Mr. White previously served as the Chief Executive Officer of iDrop, Inc., a company focused on the development and commercialization of novel ophthalmic pharmaceuticals. He developed the company’s two initial portfolio product candidates. Prior to that, as CEO of Icon Bioscience, Mr. White established a pipeline of eight ophthalmology products, including the FDA-approved product, Dexycu®, and executed multiple collaboration and partnership agreements. Mr. White was also the CEO of the Research Foundation at the University of Alabama at Birmingham, and president of Integrated Protein Technologies, a unit of Monsanto. He has held additional technical management positions, including vice president of business development and licensing, Pharmaceutical R&D for Storz Ophthalmics. Mr. White has an M.B.A. from the Virginia Commonwealth University, a B.S. in Pharmacy from the Medical College of Virginia (VCU), and a B.A. in Biology from the University of Virginia.
Omkar Joshi, PhD
Chief Technical Officer

Omkar Joshi, Ph.D., is the Chief Technical Officer at Valitor, previously serving as the company’s Senior Vice President of Technical Operations. Prior to joining Valitor, he served as Vice President of Technical Operations at Actym Therapeutics where he was a member of the Executive Leadership Team and was responsible for all aspects of technical development, manufacturing, analytical development and quality. Before Actym, Dr. Joshi held the role of Senior Director, MSAT (Manufacturing Sciences and Technology) at Bayer. In his 16-year career at Bayer, he held roles of increasing responsibility, spanning Drug Substance and Drug Product Process Development, Analytical Development and Preclinical Research. He has extensive experience working across various modalities including recombinant proteins, monoclonal antibodies, ADCs, cell therapies and gene therapies. Dr. Joshi earned a Ph.D. in Chemical Engineering from Oregon State University.
Board of Directors
Anthony Aiudi, PharmD
Anthony Aiudi, Pharm.D., MBA, has been part of the investment team at Morningside Technology Advisory since 2020. He has experience providing operational and managerial oversight to early-stage biotechnology companies. Dr. Aiudi serves on the boards of several biotechnology companies across a broad spectrum of therapeutic areas. He has also facilitated company creation, completed multiple financings for private companies, and provided operational support for portfolio companies. In 2014, Dr. Aiudi completed a Fellowship in Clinical Research at Cubist Pharmaceuticals and Northeastern University, where he was also adjunct faculty. He later was a senior clinical research scientist at Merck & Co. From late 2015-2020, he was a Director at Stealth BioTherapeutics, where he led the clinical development for numerous rare disease programs through their global Phase 3 program and supported the company through its IPO. Dr. Aiudi received his Pharm.D. and MBA from the University of Rhode Island and an MS in Finance from Northeastern University, all with highest distinctions.
Wesley Jackson, PhD
Wesley Jackson, Ph.D., has been actively involved in biotechnology entrepreneurship for over 20 years as a scientist, researcher, and innovator. Dr. Jackson was part of Valitor's founding team and helped to in-license the novel protein-biopolymer conjugation technology, which now forms the basis of Valitor’s MVP platform, from U.C. Berkeley. Since launching Valitor, he has been the principal investigator on nine NIH projects to optimize the manufacturing, characterization, and formulation of multivalent protein conjugate drugs and to evaluate their performance in relevant disease models. Prior to Valitor, Dr. Jackson was a consultant for biotech startups providing expertise in establishing and implementing preclinical and clinical development plans. Prior to his industry tenure, he was a scientist and researcher affiliated with several universities, including U.C. Berkeley, and governmental agencies, such as National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Jackson has a Ph.D. in Bioengineering from the U.C. San Francisco and U.C. Berkeley, an M.S. in Bioengineering from U.C. San Francisco, and a B.S. in Bioengineering from U.C. Berkeley.
Gregory D. Kunst
Gregory D. Kunst is the chief executive officer at Valitor. Prior to Valitor, Greg served as founder, chief executive officer and board member at Aurion Biotech, a private clinical-stage cell therapy company focused on a range of ophthalmic diseases which he sold to Alcon in 2025. Before Aurion , Greg spent six years at Glaukos Corporation, where he led the worldwide marketing, market access, reimbursement, health economics and outcomes research, government affairs, and business development teams. During his tenure at Glaukos, Greg launched four new products and led the development for multiple ophthalmology device, pharmaceutical and biologic candidates targeting corneal, glaucoma, and retinal diseases. Prior to Glaukos, Greg was global franchise director over the glaucoma surgery and retina pharmaceutical businesses at Alcon, a Novartis company. Prior to Alcon, Greg was the global head of market access at Kinetic Concepts, Inc. (Acelity Inc.) Greg is a national advisory board member of the Brigham Young University School of Life Sciences. Greg has previously been a member of the steering committee of the Gavin Herbert Eye Institute at the University of California, Irvine. Greg is an independent board member of a number of life sciences companies including Biomimetix, Pr3vent, Feliqs, and SIME Diagnostics. Greg holds an MBA from Vanderbilt University and a BS in Economics from Brigham Young University.
Gail Maderis, MBA
Gail Maderis is Chairman and former CEO of Antiva Biosciences, Inc., a venture-backed biopharmaceutical company pioneering topical therapies to treat cervical pre-cancers. Previously, Ms. Maderis led BayBio, Northern California’s life science industry organization. From 2003-2009, she served as President and CEO of Five Prime Therapeutics, a protein discovery company focused on immuno-oncology. Ms. Maderis held senior executive positions at Genzyme Corporation, including founder and president of Genzyme Molecular Oncology (GZMO). Ms. Maderis also practiced management and strategy consulting with Bain & Co. She serves on the corporate boards of DURECT (DRRX), Allarity Therapeutics (ALLR) and Antiva Biosciences, as well as on the non-profit boards of BIO (Emerging Company and Health Sections), CLS, The Termeer Foundation, and the University of California Berkeley Foundation Board of Trustees. She received a B.S. in business from U.C. Berkeley, and an MBA from Harvard Business School.
Walter Moos, PhD
Walter Moos, Ph.D. is Managing Director of Pandect Bioventures, with more than three decades of experience in executive roles in pharmaceutical and biotechnology organizations. Dr. Moos has served as CEO of ShangPharma Innovation. He has also previously served as president of SRI Biosciences (Stanford Research Institute), where he worked closely with academic, industry and government partners to bring new medicines and devices closer to market. Previously, he was chairman and CEO of MitoKor (Migenix) and a vice president at Chiron (Novartis) and Warner-Lambert/Parke-Davis (Pfizer). Dr. Moos has served on numerous boards and as an advisor to government, nonprofit and for-profit organizations, and he has held faculty positions at several major universities, including serving as an adjunct professor of pharmaceutical chemistry at the University of California, San Francisco since 1992. He has served as a consultant to industry in North America, Europe, and Asia, and has worked as an advisor or committee member for organizations including the U.S. National Academy of Sciences and Red Abbey Venture Partners. Dr. Moos has co-founded several scientific journals, co-authored and edited a number of books, and has published and patented widely. He holds an A.B. in chemistry from Harvard University and a Ph.D. in chemistry from U.C. Berkeley.
Haitao Zhu, PhD
Haitao Zhu, Ph.D., is a partner at First Spark Ventures. Prior to joining First Spark, Haitao was a Partner at SVE Capital, a Silicon Valley venture capital firm specializing in investments in early-stage biotech and med-tech companies. Before joining the venture capital world, Haitao was a group leader at Genentech, the US subsidiary of Roche, where she led drug discovery programs from inception to clinical development and evaluated many new technologies as acquisition targets. She is currently an advisor to the Spark Program in Translational Research at Stanford School of Medicine and serves on the boards of a number of startups. Throughout her career, Haitao authored numerous research papers in high profile journals, such as Nature, Cell, and Science Translational Medicine. Haitao trained as a postdoctoral fellow in Neuroscience at Stanford University and holds a Ph.D. in Biochemistry from Stony Brook University.
Technology
Valitor’s Multivalent Polymer (MVP) technology platform was created for improved therapeutic potency, durability, and safety.
Valitor’s groundbreaking technology platform is based on proprietary multivalent biopolymers that can be loaded with multiple copies of bioactive molecules. The biopolymers and bioactive molecules are interchangeable, which enables Valitor to assemble novel macromolecular entities that are engineered to overcome a multitude of specific drug design challenges for their target indications. This approach allows for independent control of multiple drug attributes, including pharmacokinetic/ pharmacodynamic properties, improved target engagement/tissue localization, therapeutic durability, and improved safety.
The initial focus of the company is to apply its technology to develop best-in-class therapies for multiple indications in ophthalmology, but the broad potential applicability of the platform can lead to rapid pipeline expansion and partnering opportunities, such as for the treatment of cancer or joint diseases.
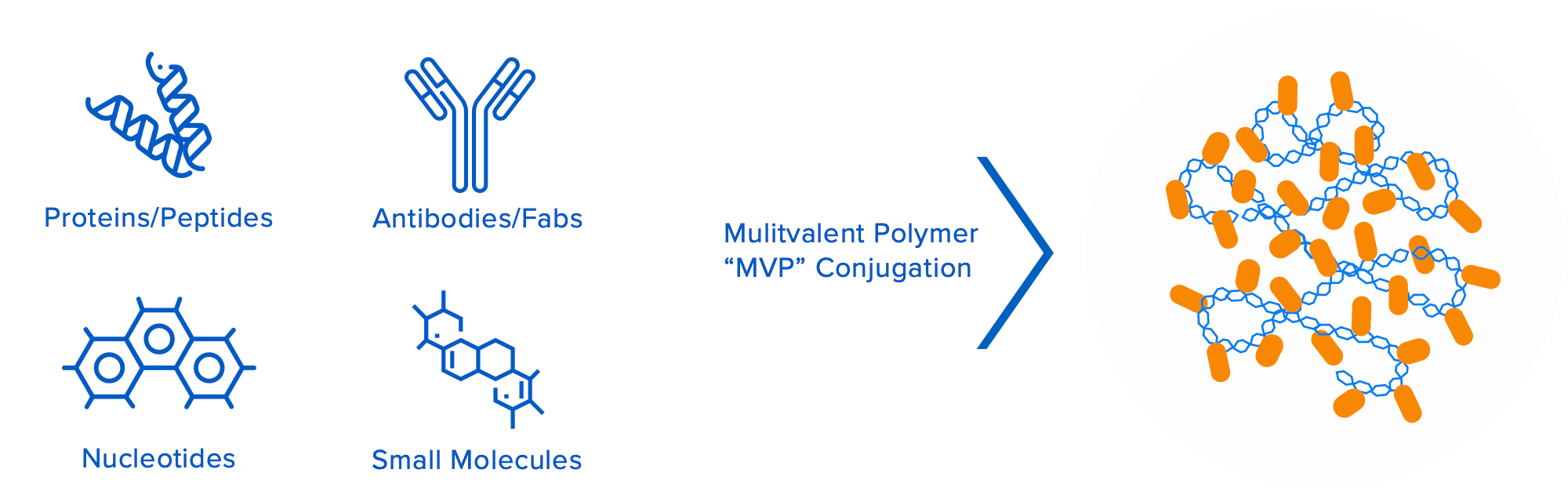
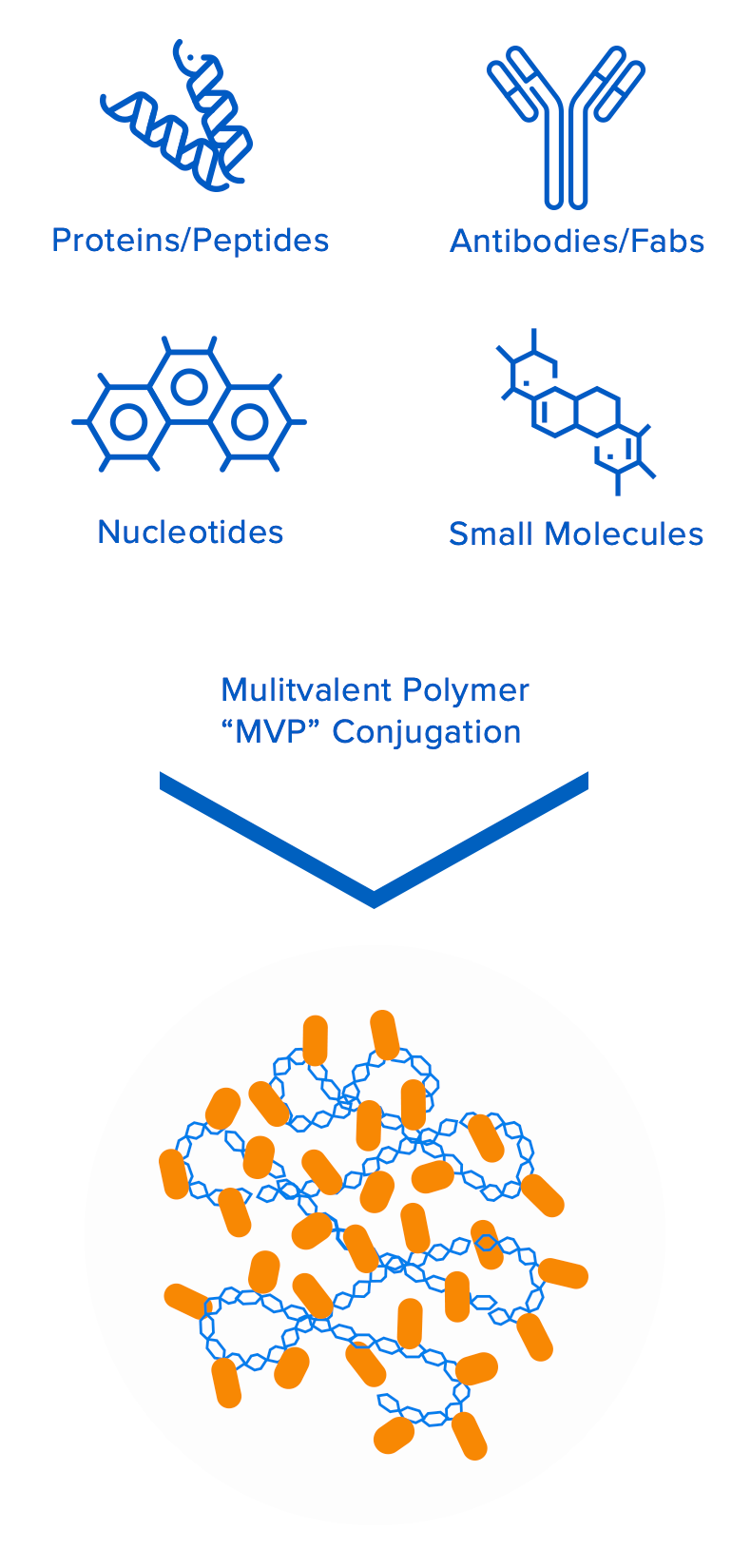
Engineered to overcome the limitations of conventional therapeutics:
Extended Target Tissue Retention (superior pharmacokinetics)
Protection from Deactivation/Reactivity (superior pharmaco-stability)
Engineered for High Potency (superior pharmacodynamics)
Pipeline
Novel compounds have shown multiple-fold increases in potency and tissue retention (half-lives) with excellent preclinical safety.
Valitor’s lead development candidate, VLTR-559, is a long-acting treatment for wet age-related macular degeneration (AMD). Wet AMD is a degenerative eye disease caused by the growth of abnormal blood vessels in the eye which leak into the retina. Anti-VEGF therapy works by blocking a protein that inhibits the disease pathology. It is the gold-standard treatment for wet AMD, providing the most effective vision protection. However, current anti-VEGF treatments require a high burden of intravitreal injections, and frequent office visits for disease monitoring are needed to prevent losses in efficacy over the long-term.
We are developing a next-generation anti-VEGF therapy that will enable reliable dosing only twice yearly, with the overall goal of improving long-term efficacy. In preclinical studies, VLTR-559 exhibited potency that was consistent with approved anti-VEGF therapies and remained in the ocular tissues three to four times longer, demonstrating unprecedented durability at the target site.
Valitor is exploring additional programs through collaborations.
VLTR-559
Wet AMD (following with DME)
- Description:
- Wet AMD (following with DME)
- Phase:
- IND-Enabling,33%
Undisclosed MOA
Pharma Collaboration 1
Undisclosed Indication
- Description:
- Undisclosed Indication
- Phase:
- Preclinical,50%
Undisclosed MOA
Pharma Collaboration 2
Undisclosed Indication
- Description:
- Undisclosed Indication
- Phase:
- Discovery,75%
Bifunctional MOA
Undisclosed Indication
- Description:
- Undisclosed Indication
- Phase:
- Discovery,50%
News
November 20, 2025
Valitor Announces Upcoming Presentation at the 15th Annual Ophthalmology Innovation Summit (OIS)
October 17, 2025
Valitor Appoints Biotech and Ophthalmic Industry Veteran Gregory D. Kunst as Chief Executive Officer
Events
Ophthalmology Futures Retina Forum
September 3, 2025
OIS Retina Innovation Summit
July 29, 2025
Select News Articles
Careers
We’re motivated by the opportunity to make an impact.
We have established a corporate culture based on collaboration, communication and creation. We are here to create new medicines for patients, meaningful experiences for employees and value for stakeholders.
For our employees, we offer an attractive compensation package along with medical, dental, and vision insurances. We also enjoy spending time together at company events and we take pride in being responsible members of the community through company-hosted volunteer work and STEM-related activities.
Job openings
We are not seeking to fill any positions at this time, but we are always interested in qualified candidates. Feel free to send your resume and cover letter to careers@valitorbio.com.










